Antimicrobial Screening System

i-Series Plus Solution Package i-Series Plus Food Safety Analyzer
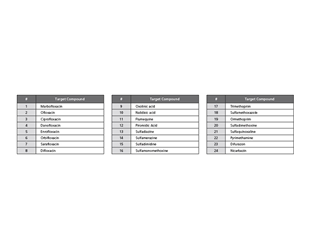
Screening of 24 Synthetic Antimicrobial Compounds That Remain in Meat Synthetic antimicrobial agents are used as animal drugs and feed additives and they are known to remain in the body of farm animals. Residue limits prescribing the safe ingestible amounts of such agents have been set so that people can safely eat meat and processed meat products. Thanks to a pretreatment method*1 that minimizes the influence of impurities in meat (beef, pork, and chicken muscle), LC system enables screening of 24 synthetic antimicrobial compounds, which are regulated in Japan, Europe, and other regions, remaining in meat. *1: Consumables for pretreatment are not included in the package. They need to be provided by the customer.
Features
-
Detects Synthetic Antimicrobial Agents of the Standard Residual Concentration with High Sensitivity
-
Quickly Confirm Screening Results in the Data Browser Window
Multiple chromatograms and automatically calculated quantitative...
News / Events
-
Shimadzu has released the Nexera MX.
Nexera MX processes twice the number of samples as conventional LCMS systems in the same amount of time.
-
“Advanced i-Series” High Performance Liquid Chromatograph
Retaining the excellent basic functions of the flagship “i-Series”, the Advanced i-Series boasts increased pressure resistance and additional functions to support remote work including working from home.
-
Shimadzu has released the ELSD-LT III Evaporative Light Scattering Detector for HPLC.
The evaporative light scattering detector (ELSD) is a general-purpose universal detector that can even detect components with no UV absorption, such as carbohydrates, lipids, surfactants, and synthetic polymers.
-
Shimadzu has released the Microsampling Device, MSW2™ Type Udck™
For Research Use Only. Not for use in diagnostic procedures.
-
Shimadzu has released Shim-pack MC PLONAS Series.
Shim-pack MC PLONAS 2.7 μm columns reduce the back pressure yet maintain the performance of a sub 2 μm fully porous particle column. The pressure limit is 40 MPa.
-
Application Note: "Analysis of Pharmaceuticals' Impurity - Regulations and Analysis for Carcinogenic Substances -"