User interviews December 2020
Our field of activity is contract research and testing for life sciences industries including pharma, biotech, nutraceuticals, environmental sciences, food&agri testing, and diagnostics.
Enhancing the Efficiency of Impurity Analysis

Question:
When impurities are detected by UV under non-volatile mobile phase conditions, does one need to adapt the method to use volatile mobile phase additives for LCMS confirmation?
Answer:
With the use of a Trap-free 2D LCMS system, no additional method development is required because mobile phases can be changed online.

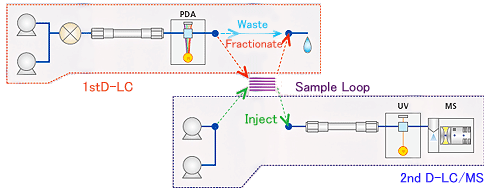
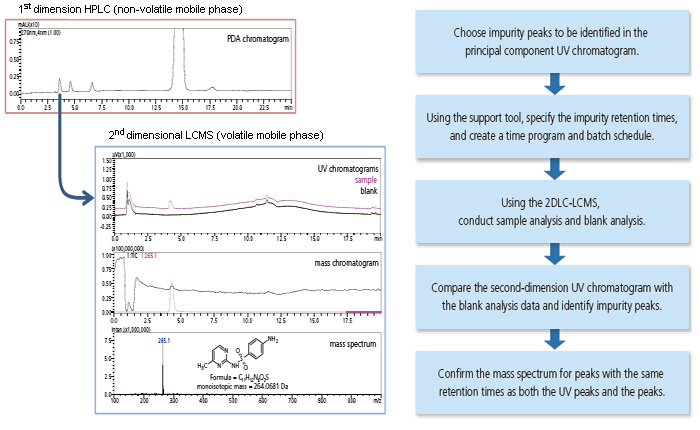
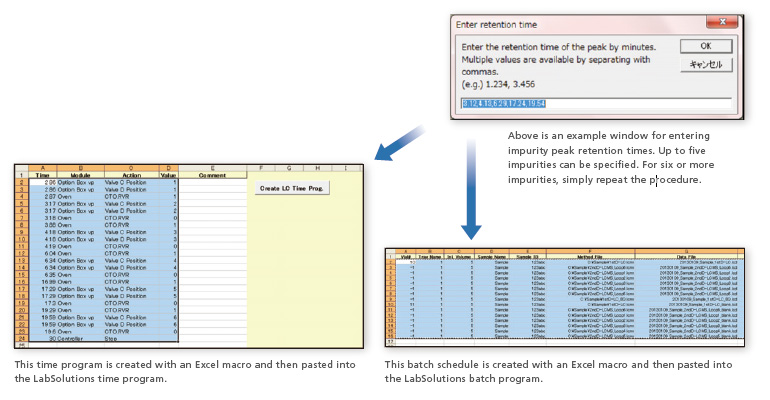
The optimal valve sequence can be constructed by entering the retention times for impurity peaks observed in the first-dimension UV chromatogram, and then a batch schedule can be created to acquire data on multiple impurities and their respective blanks.
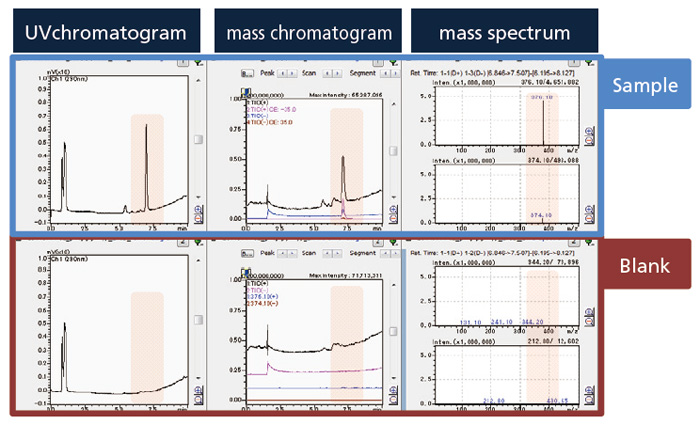
Example of sample data and blank data displayed in the data browser
Remarks and Precautions
User interviews December 2020
Our field of activity is contract research and testing for life sciences industries including pharma, biotech, nutraceuticals, environmental sciences, food&agri testing, and diagnostics.
LCMS Food Safety Application is now available.
Application Note: "Analysis of Pharmaceuticals' Impurity - Regulations and Analysis for Carcinogenic Substances -"
Application Note: "Various Analysis Techniques for Organic Acids and Examples of Their Application"
Shimadzu has released the Metabolites Method Package Suite.
Provides ready-to-use methods for over 1900 metabolites
This suite allows comprehensive analysis of over 1900 metabolites without the need for investigation of separation conditions, MRM optimization or parameter settings.
Direct Probe Ionization Mass Spectrometer - APPLICATION NOTEBOOK -