MAXima_X XRD-7000 - Applications
X-ray Diffractometer
Polycapillary Parallel-beam Optical System (Mechanical Components)
Shimadzu Analytical & Measuring Center
The term "polycapillary" indicates multiple (poly) fine glass tubes (capillaries) that guide X rays. X rays generated by a point source are accepted at a high solid angle by the polycapillaries, and emerge as a parallel beam at the opposite end. This optical system utilizes the X rays generated by the X-ray tube more efficiently than the standard (concentrating) method (the Bragg-Brentano Method), to achieve higher diffraction X ray intensities. The parallel-beam polycapillary method used in the optical system ensures that the angle of diffraction does not change due to displacements of the sample measurement surface. This characteristic allows highly sensitive and accurate measurements of samples with curved or irregular surfaces and improves the separation of the diffracted lights and shift in the angles of diffraction inherent in the concentrating method. These features of the polycapillary parallel-beam optical system permit the direct measurement of complex-shaped samples, such as mechanical components.
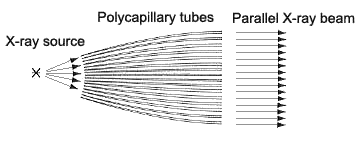
Fig. 1 Concept of the Polycapillary Parallel-beam Optical System
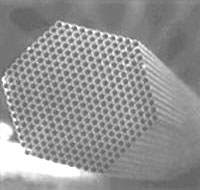
Fig. 2 SEM image of Polycapillary Tubes
Measurement of Zeolite Catalyst
he measurement of a zeolite catalyst used to purify automobile emissions is presented here as an example of the measurement of an irregular surface. As shown at the bottom of Fig. 4, the standard (concentrating) method results in a shift in the angles of diffraction and separation of the diffracted lights. The shift in the angles of diffraction to lower angles is assumed to result from superimposition with diffraction lights reflected from the inside of holes in the sample, as shown in Fig. 1. Conversely, the angular positions of the diffracted lights measured using the polycapilary parallel-beam method perfectly match ICDD database card No. 46-1600 Mg2Al4Si5O18 (represented by lines in the diagram), confirming that the measurement was unaffected by the sample shape. The high diffraction intensity allows small peaks to be captured.

Fig. 3 Photograph of the Zeolite Catalyst
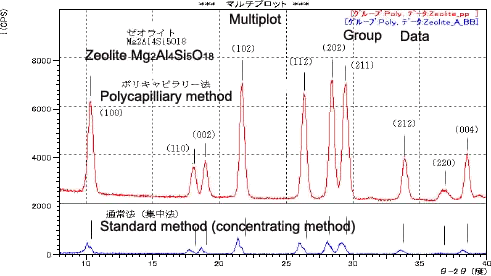
Fig. 4 Measurement Data of the Zeolite Catalyst
Measurement of a Welding Bead
Fig. 5 shows the measurement results on the corroded area of a welding bead. Oxides including Fe2O3 and FeO were detected but magnetite (Fe3O4) was the principal component of the corrosion products. The a-Fe detected close to the angle of diffraction 2q = 45°C) is thought to be the base metal. Easy quantitative identification (simple calculation using the I/Ic corundum ratio from the IC_DD database card) was conducted using search software to determine the quantitative values. This indicates that the polycapillary method extends the range of measurable samples, as measurements are unaffected by surface irregularities. The extremely high diffraction intensity attainable is expected to enhance the search accuracy.

Fig. 5 Photograph of the Welding Bead
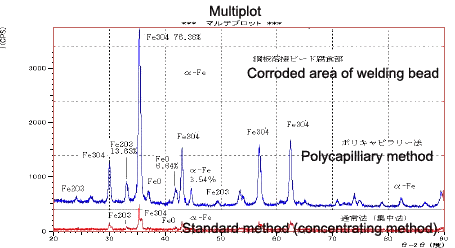
Fig. 6 Measurement Data of the Corroded Area of the Welding Bead
Measurement of Residual Austenite in Steel Balls
Fig. 7 shows quantitative measurements to determine the residual austenite in approximately 2 mm-diameter miniature steel ball bearings. A dramatic drop in diffraction intensity occurred with the standard method, as only the apex of the sample surface contributed to the diffraction due to the high curvature of the sample surface. However, the polycapillary method is able to detect diffracted lights from a wide area, permitting highly sensitive measurements that confirmed the diffracted lights for the g -(200). (220) planes (top row, Fig. 7). Quantitative analysis using the method of direct comparison obtained a mean value of 1.01% for the five diffracted lights. This result indicates that the polycapillary parallel-beam method is extremely effective for detecting small peaks from samples with highly irregular surfaces.

Fig. 7 Photograph of Steel Balls
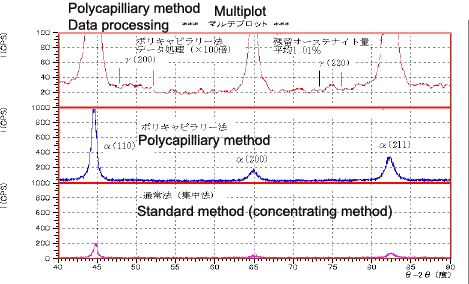
Fig. 8 Measurement Data of the Steel Balls
Measurement Examples using Polycapillary Parallel-beam Optics System (Foods, Pharmaceuticals, Organisms)
Shimadzu Analytical & Measuring Center
The term "polycapillary" indicates multiple (poly) fine glass tubes (capillaries) that guide X rays. X rays generated by a point source are accepted at a high solid angle by the polycapillaries, and emerge as a parallel beam at the opposite end. This optics system utilizes the X rays generated by the X-ray tube more efficiently than the standard (concentrating) method (the Bragg-Brentano Method), to achieve higher diffraction X ray intensities. The parallel-beam polycapillary method used by the optics system ensures that the angle of diffraction does not change due to displacements of the sample measurement surface. This characteristic allows highly sensitive and accurate measurements of samples with curved or irregular surfaces and improves the separation of the diffracted lights and shift in the angles of diffraction inherent in the concentrating method. These features of the polycapillary parallel-beam optics system permit the direct measurement of complex-shaped samples, such as foods, pharmaceuticals, or organisms.
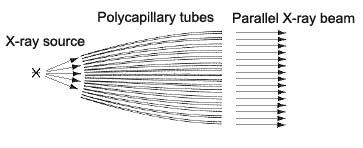
Fig. 1 Concept of the Polycapillary Parallel-beam Optics System
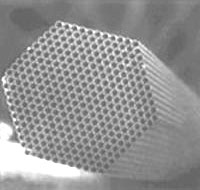
Fig. 2 SEM image of Polycapillary Tubes
Measurement of Drugs in Tablet Form
Many pharmaceuticals and organic substances are crystal polymorphs, that is, they can take different crystalline forms despite having the same chemical formula. These different crystalline forms result in different characteristics, such as efficacy or dissolution rate in the body, and can even lead to patent infringements in some cases. Consequently, quality control using X-ray diffractometer analysis is indispensable. Control of raw materials has always been required but recently control of final products is often also demanded. However, when tablets or other samples with no flat measuring surface are measured using conventional concentrating techniques, the resulting shift in the angles of diffraction or drop off in intensity makes practical measurements impossible. The analysis of such samples using the polycapillary parallel-beam method is shown in the diagram below.

Fig. 3 Appearance of Ranitidine Tablets
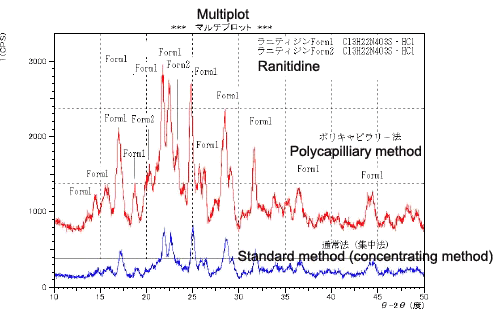
Fig. 4 Crystal Polymorph Measurement Data on Ranitidine Tablets
Fig. 4 shows measurements on the H2 antagonist ranitidine contained in gastrointestinal medications, using the polycapillary and standard methods. Form 1 is the principal component of this sample. The measurements were conducted to determine whether the crystal polymorph, Form 2, also exists. The polycapillary method confirmed the characteristic Form 2 diffracted light (near 2θ=20°), clearly indicating the existence of Form 2. The lower sensitivity of the standard (concentrating) method was unable to clearly confirm the existence of Form 2. The measurement profile obtained by the polycapillary method clearly distinguishes between the halo peaks from the amorphous areas and the peaks from the crystalline areas, and proved suitable for accurate degree of crystallinity calculations.

Fig. 5 Photograph of Tooth Appearance
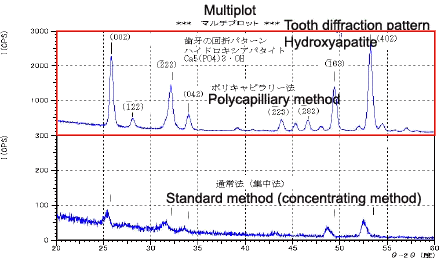
Fig. 6 Measurement of the Tooth
Measurement of Chocolate Ball
Measurement using the normal method of a chocolate ball, as shown in Fig. 7, detected virtually no diffracted lights due to crystallinity. However, the polycapillary method could clearly detect the diffracted lights of the sucrose content of the chocolate, as shown in Fig. 8. The degree of crystallinity of the sucrose was approximately 50%. The dramatic drop in diffraction intensity with the standard method is thought to occur because only the central part of the sample surface contributes to the diffraction due to the curvature of the surface.

Fig. 7 Appearance of Chocolate Ball
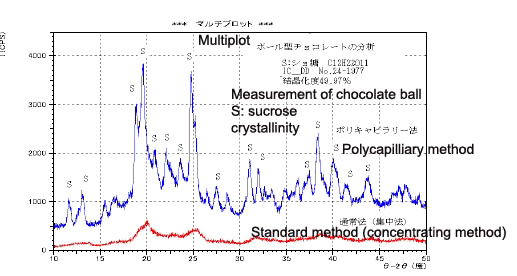
Fig. 8 Measurement of Chocolate Ball (Degree of Crystallinity)
| Applications | Creation Date |
|---|---|
|
2020-12-10 |

Most of the documents on the LITERATURE is available in PDF format. You will need Adobe Acrobat Reader to open and read PDF documents. If you do not already have Acrobat Reader, you can download it free at the Adobe's Website. Click the GET ADOBE READER icon on the left to download a free copy of Adobe Acrobat Reader.


