Effects of Sample Solvents on Peak Shape
Did this ever happen to you during reverse-phase analysis?
- Despite injecting the same volume of standard solution (methanol solvent) and test sample solution (water extract), the component peak is thicker when the standard solution is injected, for some reason.
- Separation becomes extremely poor when the injection volume of methanol solvent is doubled. Let's discuss the cause of these issues.
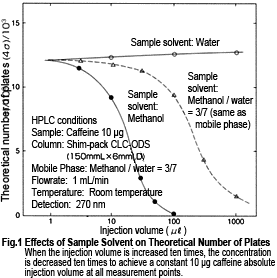
Fig. 1 shows how the theoretical plate number (N) for caffeine changes when the sample solvent is changed. If the same methanol/water (3/7) mixture as the mobile phase is used as the sample solvent, the diagram shows a clear decrease in theoretical plate number (which equates to a thicker peak) for an injection volume of 100 µL, or more.
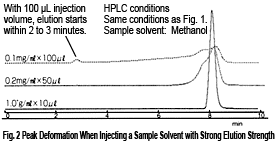
When using 100 % methanol, a decrease in theoretical plate number can be confirmed for an injection volume of only 10 µL or more, and leading occurs at higher injection volumes (Fig. 2). Then, at 100 µL, elution starts gradually from a time where almost no retention seems to occur, which results in a drop to N < 100. The sample solvent can be viewed as part of the mobile phase in the column. Therefore in this case, the sample solvent is thought to become a mobile phase with strong elution strength causing rapid migration of the components.
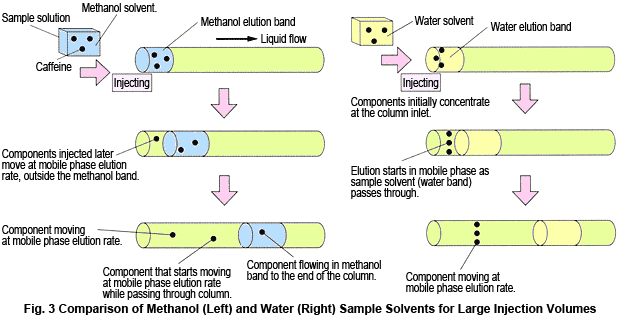
On the other hand, if 100 % water is used, no decrease in theoretical plate number could be confirmed, even for a large 1 mL injection volume. Actually, a slight increase is observed. The retention time is delayed by the equivalent of 1 mL. because the sample solvent acts as a mobile phase with weak elution strength, the caffeine initially concentrates at the column inlet and then once more starts to separate in the mobile phase. That is, it appears to be "point injected".
When the sample injection volume is comparatively large, the type of sample solvent has a significant effect on the peak shape and retention time. Take due care when developing analysis methods.