Particle Size Distribution Dependent on Principle of Measurement
The fact that particle size distribution is dependent on principle of measurement is an extremely substantive problem arising from the very concept of "particle size distribution."
"Particle size distribution" is an index (means of expression) indicating what sizes (particle size) of particles are present in what proportions (relative particle amount as a percentage where the total amount of particles is 100 %) in the sample particle group to be measured.
Volume, area, length, and quantity are used as standards (dimensions) for particle amount. However, generally, the volume standard is apparently often used.
Frequency distribution indicates in percentage the amounts of particles existing in respective particle size intervals after the range of target particle sizes is divided into separate intervals. Whereas, cumulative distribution (for particles passing the sieve) expresses the percentage of the amounts of particles of a specific particle size or below. Alternatively, cumulative distribution (for particles remaining on the sieve) expresses the percentage of the amounts of particles of a specific particle size or above. (See Fig. 1.)
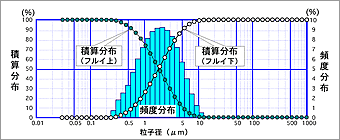
Fig. 1 Example of Particle Size Distribution
To introduce the concept of "particle size distribution", "particle size" must first be defined. The shape of almost all particles cannot be simply and quantitatively expressed as "spheres" or "cubes." Particles are complex and irregular shapes, and their particle size cannot be directly defined. This is why the indirect definition "sphere-equivalent diameter" is used. Under this definition, when a certain particle is measured based on a certain principle of measurement, the particle size of the measured particle is expressed by the diameter of a spherical body that displays the same result (i.e. measurement quantity or pattern). For example, with the "precipitation method," the particle size of the particle to be measured having the same precipitation velocity as a sphere of diameter 1 µm of the same substance as the particle to be measured is assumed to be 1 µm. However, with the "laser diffraction/scattering method," the particle size of the particle to be measured that shows the same diffracted/scattered light pattern as a 1 µm-diameter sphere is 1 µm regardless of the shape of the particle.
This means that, if the principle of measurement differs, the definition of particle size, in other words, the scale itself used as the measurement standard differs. In which case, completely different measurement results will be obtained even if the term "particle size distribution" is the same. Accordingly, in reality, we have no choice but to consider the principle of measurement itself to be a scale or a standard. For this reason, it is meaningless to scientifically or subjectively rank precision or accuracy when comparing various principles of measurement. In some aspects, the questions "Which is the right principle of measurement?" and "Which is the right God?" might both have something in common.
In selecting the principle of measurement or an analyzer, you must clearly understand the target you are yourself about to measure and the purpose of measurement, and then thoroughly examine whether or not the properties and specifications of the analyzer (e.g. measuring range, resolution and sample state during measurement) are really suited to that target and purpose of measurement.
When attempting to upgrade an existing particle size analyzer to one that operates by a different principle of measurement, it would generally be better not to assume that existing results, QC standards and other past data can be used or applied as they are. In such cases, you should provide a makeshift period for joint use of the old and new analyzers and quantitatively review standards, criteria, etc. for each, individual measurement target to ensure smooth transition.


