Methods for Separating Sugars
Liquid Chromatography
Sugars are one of the most widely occurring and abundant type of organic compounds in the natural world. A variety of types of sugar are available, such as monosaccharides, oligosaccharides, polysaccharides, neutral sugars, acidic sugars, amino sugars, sugar alcohols, and their various isomers. HPLC systems are commonly used to separate and analyze such sugars. Careful selection of an appropriate separation method and detection method is required according to the objectives. This page discusses such separation methods (primarily for neutral sugars).
Types of Separation Methods
There are five representative modes used to separate sugars, as described below. Each mode is used to separate sugars based on different mechanisms.
- ■ Size Exclusion: Size of molecules
■ Ligand Conversion: Tendency to form complexes with metal counterions
■ Partition (normal-phase): Tendency to partition in stationary phases (or aqueous phases)
■ Anion Exchange: Tendency to exchange anions
■ Borate Complex Anion Exchange: Tendency of a complex with borate to exchange anions
(However, in some cases, more than one of these modes can be used.)
Size Exclusion
This is used to separate sugars based on molecular weight. It provides a distribution of molecular weights ranging from several hundreds to several millions. Essentially, separation is based on molecular size, so components with the same molecular weight cannot be separated.
A hydrophilic polymer is used for packing material, and only water is generally used as the mobile phase. However, a salt is sometimes added to the mobile phase for ionic or other components, which can interact with the packing material.
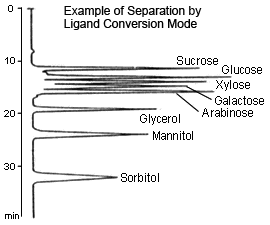
Ligand Conversion
This method uses a sulfonated polystyrene gel with a metal counterion, such as sodium (Na type), calcium (Ca type), or lead (Pb type), as a packing material and is primarily suitable for separating sugars up to disaccharides. The retention of sugars using this method is primarily based on the formation of complexes between the sugars' hydroxyl groups and metal counterions. Therefore, retention varies in relation to the stability of complexes created by exchanging sugars with the hydration water from the metal counterions. At the basis of separation is the size exclusion method, which has a molecular weight exclusion limit of about 1000, but retention may differ even for monosaccharides of the same molecular weight, depending on the type of metal counterion or the position or number of hydroxyl groups in the sugar.
With the Na type, most separation is caused by size exclusion (only glucose and fructose can be separated, for example). With the Ca type, sugar alcohols are selectively retained, whereas the retention of sugar alcohols becomes stronger and mutual separation of some monosaccharides also becomes better with the Pb type.
Since only water is used as a mobile phase, this method is environmentally friendly. On the other hand, however, the mobile phase cannot be used to control separation.
In addition, it is difficult to mutually separate disaccharides using this method. With Ca or Pb type, care must be used in setting the column temperature to 80 °C (to prevent peaks from splitting or deforming due to anomer separation).
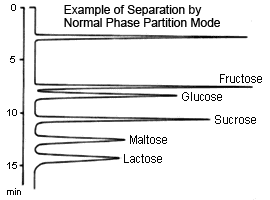
Partition (normal-phase)
This method is suitable for separating sugars ranging from monosaccharides to oligosaccharides. In particular, because it allows distinguishing between differences in individual constituent sugars in oligosaccharides, it can be used to easily separate two types of sugars.
Typically, a packing material of aminopropyl bonded to silica polymer support is used for the partition method. A mixture of acetonitrile and water is used as the mobile phase, where the higher the ratio of water, the faster the elution. In addition, the higher the molecular weight of the sugar, the longer it takes to elute.
In this method, retention of sugar is thought to be due to the partition of sugars from the water that is "condensed" in the stationary phase. However, the aldehyde radicals in sugars can react with the amino radicals in the stationary phase to create Schiff bases, which can sometimes cause significant tailing on peaks for pentasaccharides (such as arabinose and ribose) in particular. This can be inhibited by adding a salt to the mobile phase. Note also, that there is a limit to how much monosaccharides can be separated.
Anion Exchange
Since the pKa value for neutral sugars is about 12, neutral sugars can be retained in an anion exchange polymer if strongly-basic mobile phases is used. In such cases, an approximately 0.1-M sodium hydroxide solution is used as the mobile phase.
In general, sugars elute in order from monosaccharides to oligosaccharides. When combined with a gradient method that varies the concentration of sodium hydroxide, multiple components can be separated at the same time.
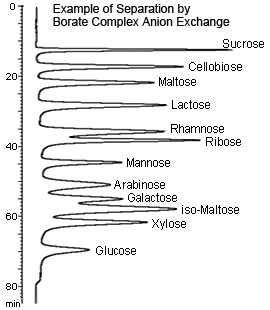
Borate Complex Anion Exchange
Sugars and other polyoxy compounds quickly react with borate or borate salts to form negatively charged complexes. In other words, sugars can be separated by anion exchange if using a borate buffer solution as the mobile phase.
This method is especially effective in separating disaccharides from monosaccharides. In particular, using a gradient method that varies the concentration and pH of the borate buffer solution enables separating multiple components efficiently.
As described above, separating sugars can be quite difficult. Target components and contaminants must be carefully considered to select the optimal method. The following technical reference material is available as a reference for analyzing sugars.
(Mk)