Preparing Buffer Solutions
The pH of the mobile phase (eluent) is adjusted to improve component separation and to extend the column life. This pH adjustment should involve not simply dripping in an acid or alkali but using buffer solutions, as much as possible. Good separation reproducibility (stability) may not be achieved if buffer solutions are not used.
A buffer solution is prepared as a combination of weak acids and their salts (sodium salts, etc.) or of weak alkalis and their salts. Common preparation methods include: 1) dripping an acid (or alkali) into an aqueous solution of a salt while measuring the pH with a pH meter and 2) making an aqueous solution of acid with the same concentration as the salt and mixing while measuring the pH with a pH meter. However, if the buffer solution is used as an HPLC mobile phase, even small errors in pH can lead to problems with separation reproducibility. Therefore, it is important to diligently inspect and calibrate any pH meter that is used. This page introduces a method that does not rely on a pH meter. The method involves weighing theoretically calculated fixed quantities of a salt and acid (or alkali) as shown in the table below. Consider the important points below.
Denoting Buffer Solutions
A buffer solution denoted, "100 mM phosphoric acid (sodium) buffer solution pH = 2.1," for example, contains phosphoric acid as the acid, sodium as the counterion, 100 mM total concentration of the phosphoric acid group, and a guaranteed buffer solution pH of 2.1.
Maximum Buffer Action Close to the Acid (or Alkali) pKa
When an acetic acid (sodium) buffer solution is prepared from 1:1 acetic acid and sodium acetate, for example, the buffer solution pH is approximately 4.7 (near the acetic acid pKa), and this is where the maximum buffer action can be obtained.
Buffer Capacity Increases as Concentration Increases
The buffer capacity of an acetic acid (sodium) buffer solution is larger at 100 mM concentration than at 10 mM, for example. However, precipitation occurs more readily at higher concentrations.
Beware of Salt Solubility and Precipitation
The salt solubility depends on the type of salt, such as potassium salt or sodium salt. Salts precipitate out more readily when an organic solvent is mixed in.
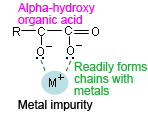
In addition, avoid using buffer solutions based on organic acids (carboxylic acid) as much as possible for highly sensitive analysis at short UV wavelengths. Consider the various analytical conditions and use an appropriate buffer solution, such as an organic acid with a hydroxyl group at the α position (see Supplement) to restrict the effects of metal impurity ions. (J.Ma,Y.Eg)
|
Reference: |
1) LCtalk Vol. 29, 8 pKa and Dissociation Equilibrium, 2) LCtalk Vol. 26, 11, 3) LCtalk Vol. 40, 4 Preparing the Mobile Phase. |
Methods to Prepare Buffer Solutions
| 100 mM phosphoric acid (sodium) buffer solution (pH=2.1) Sodium dihydrogen phosphate dihydrate (M.W.=156.01)..50 mmol (7.8 g) Phosphoric acid (85 %, 14.7 mol/L).........................50 mmol (3.4 mL) Add water to make up to 1 L. |
| 10 mM phosphoric acid (sodium) buffer solution (pH=2.6) Sodium dihydrogen phosphate dihydrate (M.W.=156.01)..5 mmol (0.78 g) Phosphoric acid (85 %, 14.7 mol/L).........................5 mmol (0.34 mL) Add water to make up to 1 L. (Alternatively, dilute 100 mM phosphoric acid (sodium) buffer solution (pH=2.1) ten times.) |
| 50 mM phosphoric acid (sodium) buffer solution (pH=2.8) Sodium dihydrogen phosphate dihydrate (M.W.=156.01)..40 mmol (6.24 g) Phosphoric acid (85 %, 14.7 mol/L).........................10 mmol (0.68 mL) Add water to make up to 1 L. |
| 100 mM phosphoric acid (sodium) buffer solution (pH=6.8) Sodium dihydrogen phosphate dihydrate (M.W.=156.01)..50 mmol (7.8 g) Sodium dihydrogen phosphate 12-hydrate (M.W.=358.14)..50 mmol (17.9 g) Add water to make up to 1 L. |
| 10 mM phosphoric acid (sodium) buffer solution (pH=6.9) Sodium dihydrogen phosphate dihydrate (M.W.=156.01)..5 mmol (0.78 g) Sodium dihydrogen phosphate 12-hydrate (M.W.=358.14)..5 mmol (1.79 g) Add water to make up to 1 L. (Alternatively, dilute 100 mM phosphoric acid (sodium) buffer solution (pH=6.8) ten times.) |
| 20 mM citric acid (sodium) buffer solution (pH=3.1) Citric Acid Monohydrate (M.W.=210.14)...............16.7 mmol (3.51 g) Trisodium Citrate Dihydrate (M.W.=294.10)..3.3 mmol (0.97 g) Add water to make up to 1 L. |
| 20 mM citric acid (sodium) buffer solution (pH=4.6) Citric Acid Monohydrate (M.W.=210.14)...............10 mmol (2.1 g) Trisodium Citrate Dihydrate (M.W.=294.10)..10 mmol (2.94 g) Add water to make up to 1 L. |
| 10 mM tartaric acid (sodium) buffer solution (pH=2.9) Tartaric acid (M.W.=150.09)..........................7.5 mmol (1.13 g) Sodium tartrate dihydrate (M.W.=230.08)........2.5 mmol (0.58 g) Add water to make up to 1 L. |
| 10 mM tartaric acid (sodium) buffer solution (pH=4.2) Tartaric acid (M.W.=150.09)..........................2.5 mmol (0.375 g) Sodium tart rate dihydrate (M.W.=230.08)........7.5 mmol (1.726 g) Add water to make up to 1 L. |
| 20mM (acetic acid) ethanolamine buffer solution pH=9.6 Monoethanolamine (M.W.=61.87, d=1.017)...20 mmol (1.22 mL) Acetic acid (glacial acetic acid, 17.4 mol/L)..................10 mmol (0.575 mL) Add water to make up to 1 L. |
| 100 mM acetic acid (sodium) buffer solution (pH=4.7) Acetic acid (glacial acetic acid) (99.5 %, 17.4 mol/L)..................50 mmol (2.87 mL) Sodium acetate trihydrate (M.W.=136.08)........50 mmol (6.80 g) Add water to make up to 1 L. |
| 100 mM boric acid (potassium) buffer solution (pH=9.1) Boric acid (M.W.=61.83)...............100 mmol (6.18 g) Potassium hydroxide (M.W.=56.11)...............50 mmol (2.81 g) Add water to make up to 1 L. |
| 100 mM boric acid (sodium) buffer solution (pH=9.1) Boric acid (M.W.=61.83)...............100 mmol (6.18 g) Sodium hydroxide (M.W.=40.00)...............50 mmol (2.00 g) Add water to make up to 1 L. |
Supplement
An organic acid with a hydroxyl group (citric acid, tartaric acid, etc.) at the α position has a "crab-claw" structure that readily forms chains with metal impurities in the mobile phase.
Shimadzu analytical balance AP Series
Faster Response and Higher Stability
Shimadzu analytical balances boast a one-piece UniBloc weighing sensor, which is now even more advanced.
Buffer Solution Preparation Mode
- Recipes for 13 commonly used buffer solutions are included as standard
Preparation recipes for commonly used buffer solutions are provided as standard - Instructions are shown on the display
The target weighing value is shown on the display and analog bar in order to compare the target with the current weight. Manual calculation is not needed. - Record function
Record output with date, time and operator name.



